a. State of the art and objectives
Metastable states represent a growing, interdisciplinary research field1 with yet unknown basic principles and many similarities to the biological world.2 It is known, however, that the collective nature of metastability, the associated slow kinetics and the complex phase space are related to the existence of long range forces and to strong spatial and temporal correlations.3,4 Under equilibrium conditions, often unattainable, the number of self-assembling structures is limited and is given by equilibrium thermodynamics. On the contrary, under non-equilibrium conditions (for example, with the use of external fields or with fine tuning of the molecular parameters such as the number of reacting units), a plethora of new self-assembling structures is formed.5 These characteristics make soft materials of growing interest with many new applications and scientific surprises.6
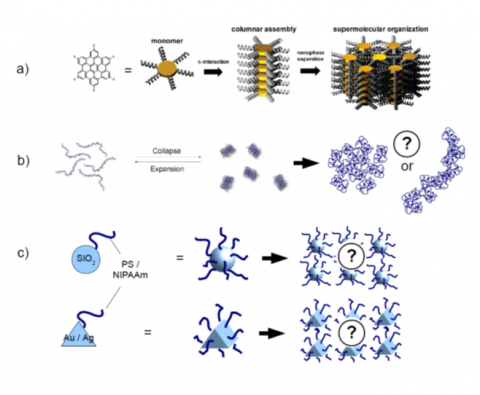
Controlling self-assembly in soft materials in- and away- from equilibrium is one of the 25 “what don’t we know” questions posed by Science at its 125 years birthday.7 The principal focus of the proposed work is along these lines, i.e. finding the basic underlying principles that give rise to self-assembly in a range of systems where structural and kinetic frustration is provided by different means. This ambitious but realistic approach requires a methodology that includes the synthesis, the structural and dynamical characterization and simulation in a number of systems exemplifying different types of interactions. These encompass molecular, supramolecular and mesoscopic systems (schematically depicted in Figure 1) with important potential applications. In addition, it requires the implementation of different but complementary techniques with high spatial and temporal resolution, operating over broad space and time scales.
Figure 1.
A Zoo of self-assemblies:
(a) nano-graphenes to (b) supramolecular systems and to (c) mesoscopic particles. In all three cases the investigation of the self-assembly and dynamics resulting from metastable states, with important technological applications, are made for the first time to this extent.
·
Molecular systems: Discotic Liquid Crystals of nano-graphenes with enthalpic/entropic interactions
In one direction, molecular discotic liquid crystals (DLC) based on nano-graphenes will be synthesized (synthesis by the External Research Team (ERT) member of RT1, Prof. K. Müllen, Mainz) and studied with respect to their metastability. Discotic liquid crystals8, consisting of rigid disk-shaped aromatic cores and disordered alkyl substituents tend to organize into columnar supramolecular structures. Alkyl substituted hexabenzocoronenes (HBC),9 in particular, were found to be very promising as active semiconductors in organic field-effect transistors and photovoltaic devices. A key factor in the design of HBCs for electronic applications is the high one-dimensional charge carrier mobility that is approaching the value for the intersheet mobility in graphite. The origin of this favorable mobility is the optimized π*-π* overlap of the aromatic cores. On the other hand, the purpose of the alkyl chains is to introduce solubility, proccessability and a rich thermotropic behavior. However, as pointed out by de Gennes10 already in 1983, the incommensurability of flexible aliphatic side chains and rigid aromatic cores poses a packing problem. This can lead to density fluctuations along the columns that can be described as a heterogeneous disorder composed of regions of high order interrupted by defects. Applications of DLC as electronic devices rely on optimal stacking of the aromatic cores that allows for charge carrier mobility along the columnar axis acting as molecular wires. Considering the potential application of DLCs as self-healing active semiconductors, the presence of defects limits the one-dimensional charge transport. For these reasons, knowledge of structural disorder in DLCs in terms of the origin of the defects, their characteristic length, and associated dynamics is essential. The origin of metastability in DLCs is the existence of different competing nanophases (with the hexagonal liquid crystalline Colh and crystalline Cr phases being the more prominent), the presence of defected “phases” and of a glass “transition”.11,12Independent from the origin of metastability, these systems are expected to be described by the usual thermodynamic (pressure-temperature) phase diagrams.
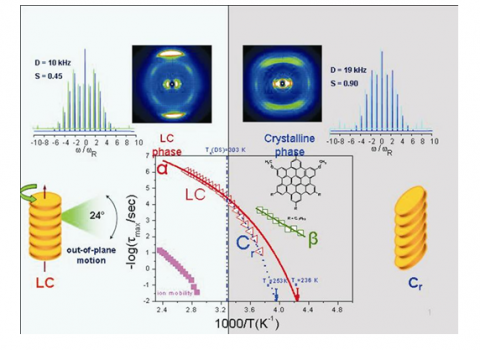
Progress in this field has been through both structural and dynamics investigations. With respect to the structure, studies11,13,14 emphasized the anisotropy in the thermal expansion reflecting the anisotropic molecular interactions; intra vs. inter-columnar, reflecting π*-π* stacking and van der Waals interactions, respectively. To this end, members of RT1 have recently shown11 that HBCs possess a large thermal expansion within the Colh phase but show thermal contraction within the Cr phase as a result of the increasing disk tilt with respect to the columnar axis (improved packing). With respect to the dynamics, earlier investigations13 identified the main α-process as reflecting the axial motion of disks around the columnar axis. More recently, concerted efforts12,14,15 by site-specific NMR and dielectric spectroscopy in some DLCs have addressed the disk dynamics in both phases (Figure 2). “Fast” and “slow” dynamics were found associated with the in- and out-of plane axial motions and with a collective re-organization of disks within the columns that result in the complete relaxation of the dipole moment. In addition, a recent investigation16 reported on the possibility of studying the kinetic pathways of the Colh to Cr phase transformation. A systematic investigation of discotic liquid crystals with respect to the nanoscale self-assembly, the molecular dynamics, the stability and metastability of phases requires a combination of model systems bearing different core sizes and dipoles directly attached to the cores as well as different microscopic techniques that are sensitive probes of the time-scale and geometry of motion. Furthermore, additional intensive thermodynamic variables are needed (i.e., pressure17, electric, magnetic, mechanical fields) for constructing the corresponding phase diagrams and for exploring the -yet unknown- phases away from equilibrium.
Figure 2.
Library of dynamics in DLCs: (Top): X-ray images corresponding to the Colh (left) and Cr (right) phases; (bottom): Disk dynamics in a dipole substituted HBC within the two phases; (upper corners): Heteronuclear NMR probing the dynamic order parameter in the two phases.15
In this work, RT1, RT3 and RT4 investigate a series of new DLCs bearing different cores (coronenes vs. HBCs), different side chains and different dipoles directly attached to the cores with respect to (i) the self-assembly (with X-ray scattering, differential scanning calorimetry, polarizing optical microscopy, IR, theory and simulation), (ii) the dynamics (with simulation, dielectric spectroscopy as a function of temperature and pressure and several advanced NMR techniques, the latter with the ERT member Prof. H.W. Spiess, Mainz), (iii) the phase state (with the application of pressure), (iv) the pathways of phase transformation (kinetics) by means of X-ray scattering, IR and dielectric spectroscopy and (v) the effects of external non-linear fields. The identification of structural defects and their associated dynamics, pertinent to the large scale organization needed for electronic applications, the equilibrium phase diagrams, the way that these phases are formed, and the application of external fields driving DLCs away from the equilibrium are addressed for the first time to this extent.
In a second direction, we propose to investigate novel supramolecular and mesoscopic materials that, contrary to DLCs, are not expected to be described by the classical phase diagrams. This area, apart from the characteristics of the structural units, includes two additional intensive variables: concentration and external directing fields.
· Self-assembly and dynamics of supramolecular systems: tailoring directional interactions
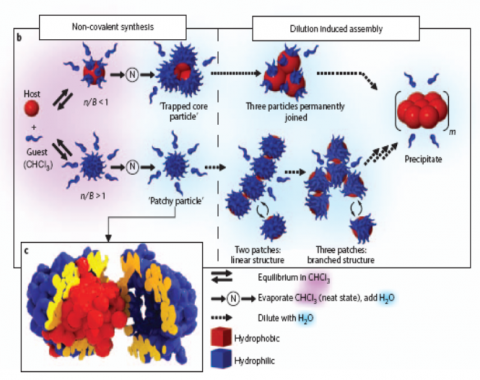
Here the detailed topology of the forces between the constituent units determines the pathways along which soft matter self-assembles into hierarchical structures.4,18,19 The design of structural units with built-in interaction directionality has opened new routes in the field of self-assembly and led to novel nanostructures such as Janus,5 patchy particles20,21 and colloidal assemblies.22,23 Based on recent computer simulations 24,25 assuming directional attractive interactions, a plethora of complex self-assemblies are anticipated, in contrast to the ingeniously controlled organization in the biological world; protein crystallization occurs whenever there is a functional need. In the process of self-organization, the equilibrium structure is often frustrated due to either structural or kinetic incompatibility between initial and final states thereby leading to non-equilibrium structures. The detailed understanding of the leading role of directional interactions per structural unit, the so-called multivalency (the particle analogue to the atoms), is a precondition to conceive not only the aggregation of proteins26 but also a wealth of transition phenomena ranging from re-entrance transitions in soft matter27 and organization of nanoparticles in nanowires28 to preparation of single chain nanoparticles.29 An example of the large impact that multivalency can exert on the aggregation of soft nanoparticles30 with controlled patchiness heterogeneity (hydrophilic vs. hydrophobic nature) is schematically shown in Figure 3. The structures in the left side of Fig. 3b represent states in thermodynamic equilibrium between the host and the guest molecules whereas the structures in the right panel are strongly depend on both system inherent properties, such as equilibrium constants and the guest exchange rates.
Figure 3. Multivalency induced self-assembly:21The fifth-generation urea-adamantyl poly(propylene imine) dendrimer (host) and the ureido acetic acid (guest). Binding is governed by acid–base and hydrogen-bonding directional interactions. (b) Host and guest are mixed in chloroform (pre-complex) and via the neat state transferred to water. Depending on the ratio of guest molecules and binding sites (n/B), a small complex with, on average, three host molecules trapped in the core (n/B<1) is formed (‘trapped core particle’) or a stable guest–host complex (n/B>1) is obtained in H2O (‘patchy particle’). Dilution (with H2O) can have a huge effect: no gradual change in the structure of the ‘trapped core particle’ versus a range of reversible equilibrium for the “patchy particles”. (c) Molecular model of the patchy particle. The host molecule forms the core of the particle (red) and the guest molecules (blue) form a protective shell.
In this work, in a targeted effort members of RT3, RT4 and RT1 explore the basic principles underlying the creation of supramolecular structures, by studying reactive (multivalent) copolymers that are based on polynorbornene with protected units of 2-ureido-pyrimidone (UPy) (synthesis by the ERT member, Prof. E.W. Meijer, Eindhoven). This choice is based on (a) their bio-mimetic self-assembly through directed hydrogen bonding, (b) the possibility of both intra- and inter-molecular interactions, (c) the in-situ activation of the interactions through photochemical removal of the protecting groups, (d) the controlled, protein-like, self-assembly both in dilute and concentrated solutions and (e) the possibility of employing simulations and suitable models to predict the possible nanostructures. Our aim here is to understand how the rich self-assembly is influenced by the molecular characteristics, the concentration and the dynamics. Identifying the exact structure in such complex systems requires the use of advanced scattering techniques and cryo-TEM (in collaboration with the ERT member M. Bockstaller). The fruitful collaboration21,31,32 established between the RT3 and the ERT members will be advantageous for the successful expedition of the proposed work with bright future ahead.33
· Mesoscopic Colloidal systems: tunable osmotic interactions and kinetic arrest
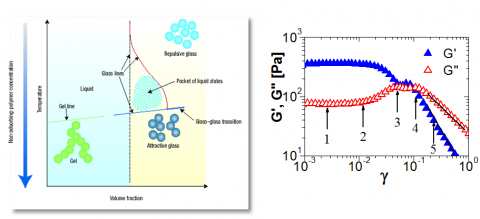
Bridging the two typical classes of soft matter (polymers/colloids) provides a new platform for the interplay of antagonistic forces of entropic, excluded volume and enthalpic origin. Controlled interactions and size polydispersity in these hybrid systems can induce kinetic arrest that leads to non-equilibrium states such as glasses and gels34-37. In a mixture of hard sphere colloids and non-absorbing linear polymer chains exemplifying an ideal system, the common “repulsive” glass of hard sphere colloidal suspension (Figure 4) is observed. In the presence of polymer chains with radius of gyration much smaller than the particle radius, the interactions turn to attractive and the activated depletion effect decreases the mechanical stability of the glassy phase that undergoes a re-entrant transition to the fluid state within a range of polymer concentration. At high polymer fractions a new “attractive” glassy state is observed (Fig. 4). In contrast to the classical thermodynamic phase diagram of DLC, the presence of different competing interactions in mesoscopic soft matter systems leads to a rich phase diagram that in addition to the fluid phase encompasses gel and different glassy states.34-37 As non-equilibrium glassy states are intimately bound to physical aging with non-ergodicity behavior, a complex rheological response is anticipated. A typical example is shown in Fig. 4, where a colloidal glass undergoes an oscillatory shearing at constant frequency and increasing strain amplitude. Whereas at low strains the glass remains intact (the storage modulus G’ exceeds the loss modulus G’’), a further increase of strain gives rise to various, not fully understood yet, structural rearrangements (vertical arrows in Fig.4) and eventually the melting of the glass (G’’>G’ with nearly constant slopes). The identification of the structures and the underlying mechanisms for the different phase transitions is currently a very active but premature research field and promise lies ahead. Significant advances in the field of glass and other kinetic transitions will allow tailoring the flow properties of soft colloids for specific applications.
Figure 4. Tuning the phase diagram and rheology: (Left) Phase diagram of hybrid mixture of hard spheres-polymer-solvent. The interactions can be turned from repulsive to attractive via variation of the composition leading to different phases within a range of volume fraction.36 (Right) Shear-induced flow under oscillatory strain reveals the different regimes for the storage (G’) and loss (G’’) moduli corresponding to structural re-arrangements (1 to 5) in a suspension of a multi-arm star polymer with 128 arms.35
Access to delicate chemistry for the synthesis of designed hybrid systems is very advantageous for the integrated approach of the proposal. In this work we propose to investigate two hybrid systems/particles: (i) spherical core (SiO2) – shell (polystyrene (PS) or poly(N-isopropylacrylamide) (PNIPAAm)) particles and (ii) low dimensionality (rods, plates) Au and Ag particles with surface coverage (PS or PNIPAAm) of 10-50 nm in thickness and with important biomedical (biosensing) and optoelectronic (plasmonics) applications (synthesis by RT2 and the ERT member, K. Matyjaszewski, Pittsburgh). The origin of metastability in such mesoscopic systems are dynamic transitions that lead to kinetic arrest (loss of their ability to flow). Important open questions38 are how the (controlled) density of surface coverage, the size, the shape and surface functionalization affect the inter-particle potential energy, which in turn affects the self-assembly, the kinetic frustration and the dynamic response to external fields (shear, electric field). We will investigate, in particular, states away from equilibrium in the presence of solvent but also within compatible polymeric matrices, where differences in the dynamic response and interactions are maximized. The interrelation between the synthetic protocol of the mesoscopic structures with the final morphology will be studied in detail (in collaboration with the ERT member, Prof. M. Bockstaller). The fruitful collaboration39 between the RT3 and the ERT member MB will be advantageous for the successful expedition of the proposed work. In addition, recent progress by RT2 has been made towards the synthesis of polymer coated core-shell nanoparticles bearing inert (SiO2-PS, Au-PS) or temperature-responsive (SiO2-PNIPAAm, Au-PNIPAAm) polymer shells via surface-initiated polymerization. The “grafting from” technique was used which involves the direct growth of the polymer chains from the inorganic surface via a chemically grafted monolayer of the initiating groups. The polymer was prepared using controlled/living polymerization methods (atom transfer radical polymerization, ATRP) which allow to accurately control the polymer chain length and molecular weight distribution as well as the polymer grafting density (chains/nm2).40 Hybrid core-shell nanoparticles with a SiO2 core and a poly(methyl methacrylate) and poly(2-dimethylaminoethyl methacrylate) shell have been also prepared following this protocol.
Progress beyond the state of the art
The proposed work refers to a new research direction that is now followed internationally.7 It concerns understanding the basic underlying principles that give rise to self-assembly and metastability in systems where structural and kinetic frustration is provided by different means. The ultimate goal is to control and design specific structures. This interdisciplinary research being at the forefront of the science of soft matter requires the participation of researchers with broad experience, complementarity of interests (theory/experiment), international mobility, established collaboration (several joint publications of the researchers) as well as the strong collaboration with some well-known research teams internationally. Investing in young and promising researchers and attracting scientists from abroad represent - given the current situation - the biggest contribution to the country. The exploitation of the results will be made with filing patents, for example, in computational codes and methodologies and if possible, with new small to medium size enterprises in the areas of microelectronics, coatings and others. This is important for countries like Greece, with only small chemical industry, and has a great added value.
Specifically, the experimental results will have immediate academic and technological exploitation. From the pure academic side, research on molecular discotic liquid crystals (DLCs), will lead to the understanding of the role of structural defects as well as of their dynamics in the presence and absence of strong external fields. From a technological side, the research results and methodology could be implemented by Industry for the design of new DLCs with optimized conductivities and mobilities of charge carriers in field effect transistors (FTE) and in photovoltaic devices. In the supramolecular systems, the possibility to predict and control the final structures could be exploited in biomedical applications, i.e., in controlled drug release, self-healing of soft materials and boost the new class of polymers based on non-covalent bonding. Lastly, understanding the mechanism and controlling the final mesoscopic and nanoscopic particles opens new opportunities for exploitation, for example, in coatings, sensors, additives in commodity plastics (for controlling the viscoelastic response), with new possibilities for surface coverage and with filling patents with the corresponding computational codes.
The dissemination of results will be made with publications in highly ranked international journals, with presentations in national and international conferences as well as with presentation of the pertinent findings to Industrial collaborators. Furthermore, the proposed research is important for the career development of 7 young researchers both in Academia and in Industry. For one, the integrated nature of the research (spanning synthesis, characterization, structure analysis, property measurement and theory/simulation, all leading to the elucidation of governing structure-property relationships) invariably broadens the background of students and better enables them to set up a similarly integrated laboratory at the outset of their independent careers.
. Conclusions .
META-ASSEMBLY is positioned at the interface of two well-established hitherto not cross-fertilizing fields with strong impact to microelectronics and the biological world. It involves novel and unconventional fabrication and characterization strategies that will enable us to achieve a fundamental understanding of the underlying mechanisms controlling the self-assembly of soft matter systems and associated phase and state transitions. This unified research approach will open up new routes for both scientific and technological research by tackling simultaneously structural, thermodynamic and kinetic issues over a broad spatio-temporal range. The research program will be implemented by an interdisciplinary team headed by the PI, 3 complementary research groups, 5 international leading scientists, 2 renowned visiting scientists and 7 PhD students. We envisage significantly contributing to the new emerging field of metastable soft matter, where the many outstanding challenges are only now just being addressed.
. References .
[1] Debenedetti, P.G. Metastable Liquids: Concepts and Principles (Princeton Univ. Press. 1996).
[2] Muthukumar, M.; Ober, C.K.; Thomas, E.L. Science 277, 1225 (1997).
[3] Sijbesma, R.P.; Bijer, F.B.; Brunsveld, L.; Meijer, E.W. Science 278, 1601 (1997).
[4] Breen, T.L.; Tien, J.; Oliver, S.R.; Hadjik, T.; Whitesides, G.M. Science 284, 948 (1999).
[5] Roh, K.H.; Martin, D.C.; Lahann, J. Nature Mater. 4, 759 (2005).
[6] Grzelczak, M.; Vermant,J.; Furst, E.M.; Liz-Marzán, L.M. ACS Nano 4, 3591 (2010)
[7] Kennedy, D.; Norman, C. Science 309, 75 (2005); Service R.F. Science 309, 95 (2005).
[8] Kumar, S. Chem. Soc. Rev. 35, 83 (2006).
[9] Wu, J.; Pisula, W.; Muellen, K. Chem. Rev. 107, 718 (2007).
[10] de Gennes P.G. J. de Physique Letters 44, L-657 (1983).
[11] Grigoriadis, C.; Haase, N.; Butt, H-J.; Müllen, K.; Floudas, G. Adv. Mater. 22, 1403 (2010).
[12] Elmahdy, M.M.; Floudas, G.; Mondeshki, M. et al. Phys. Rev. Lett. 100, 107801 (2008).
[13] Möller, M.; Wendorff, J.H.; Werth, M.; Spiess, H.W. J. Non- Cryst. Solids 170, 295 (1994).
[14] Tasios, N. ; Grigoriadis, C.; Hansen, M.R. et al. J. Am. Chem. Soc. 132, 7478 (2010).
[15] Elmahdy, M.M.; Dou, X.; Mondeshki, M.; Floudas, G. et al. J. Am. Chem. Soc. 130, 5311 (2008).
[16] Elmahdy, M.M.; Mondeshki, M.; Dou, X., et al. J. Chem. Phys. 131, 114704 (2009).
[17] Floudas,G. et.al, Molecular Dynamics of Glass-Forming Systems. Effects of Pressure (Springer, 2011).
[18] Alivisatos, A.P. et al. Nature 382, 609 (1996).
[19] Wojtecki, R.J.; Meador, M.A.; Rowan, S.J. Nature Materials 10,15 (2011)
[20] Christian, D.A. ; Liu, A.; Discher, D.E. et al. Nature Materials 8, 843 (2009).
[21] Herman, T.M.; Broeren, M.A.C.; Gomopoulos, N. et al. Nature Nanotechnology 4, 721 (2009).
[22] Manoharan, V.N.; Elsesser, M.T.; Pine, D.J. Science 301, 483 (2003).
[23] Wagner, C.S.; Lu, Y.; Wittemann, A. Langmuir 24, 12126 (2008).
[24] Zhanng, Z.; Glotzer, S.C. Nano Lett. 4, 1407 (2004).
[25] Bianchi, E.; Largo, J.; Tartaglia, R.; Zaccarelli, E.; Sciortino, F. Phys. Rev. Lett. 100, 168301 (2008).
[26] Pappu, R.V.; Wang, X.; Vitalis, A.; Crick, S.L. Arch. Biochem. Biophys. 469, 132 (2008).
[27] Zhang, F. et al. Phys. Rev. Lett. 101, 125701 (2008).
[28] Tang, Z.; Kotov, N.A.; Giersig, M. Science 297, 237 (2002).
[29] Foster, E.J.; Berda, E.B.; Meijer E.W. J. Am. Chem. Soc. 131, 6964 (2009).
[30] Russo, J.; Tavares, J.M.; Teixeira, P.I.C.; Sciortino, F. Phys. Rev. Lett. 106,085703 (2011)
[31] Versteegen, R.M.; van Beek, D.J.M.; Sijbesma, R.P. et al. J. Am. Chem. Soc. 127,13862 (2005).
[32] Hermans, T.M.; Broeren, M.A.C.; Gomopoulos, N. et al. J. Am. Chem. Soc. 129,15631 (2007).
[33] Burnworth, M.; Tang, L.; Kumpfer, J.R. et al. Nature 472, 335 (2011)
[34] Sciortino F., Nature Materials 1, 145 (2002).
[35] Mayer, C.; Zaccarelli, E.; Stiakakis, E.; Likos C.N. et al. Nature Materials, 7, 780 (2008).
[36] Zaccarelli, E.; Valeriani, C.; Sanz, E. et al. Phys. Rev. Lett. 103, 135704 (2009).
[37] Pham, K. N., Puertas, A. M., Bergenholtz, J. et al. Science 296, 104 (2002).
[38] Akcora, R.; Liu, H.; Kumar, S.K. et al. Nature Materials 8, 354 (2009).
[39] Voudouris, P.; Choi, J.; Dong, H.; Bockstaller, M.R. et al. Macromolecules 42, 2721 (2009).
[40] Mateescu, A.; Ye, J.; Narain, R.; Vamvakaki, M. Soft Matter 5, 1621 (2009).
[41] Vogel, N.; de Viguere, L.; Jonas,U. et al. Adv. Funct. Mat. (DOI:1002/adfm2011000414).
[42] Klein, O.C.; Jonas,U.; Muellen K.; Vlassopoulos, D. Soft Matter (DOI: 10.1039/C1SMO53570.)
[43] Gomopoulos, N.; Saini, G.; Efremov, M. et al. Macromolecules 43,1551 (2010).
[44] Raccis, R.; Nikoubashman, Likos, C.N.; Fytas. G. et al. ACS Nano (DOI:10.1021/nn200767xx).
[45] Plum, M.; Steffen, W.; Fytas, G.; Knoll, W.; Menges, B. Optics Express 177, 10364 (2009).
[46] Marcon, V.; Vehoff, T.; Kirkpatrick, J. et al. D. J. Chem. Phys. 129, 094505 (2008).
[47] Megariotis, G.; Vyrkou, A.; Leygue, A.; Theodorou, D.N. Ind. Eng. Chem. 50, 546 (2011).
[48] Romanos, N.A.; Theodorou, D.N. Macromolecules, 43, 5455 (2010).
[49] Doxastakis, M.; Theodorou, D.N.; Fytas, G. et al. J. Chem. Phys. 119, 6883 (2003).
[50] Ahumada, O. ; Theodorou, D.N.; Triolo, A. et al. Macromolecules 35, 7110 (2002).
[51] Reith, D.; Pütz, M.; Müller-Plathe, F. J. Comput. Chem. 24, 1624 (2003).
[52] Spyriouni, T.; Tzoumanekas, C.; Theodorou, D. N. et al. Macromolecules 40, 3876 (2007).
[53] Izvekov, S.; Voth, G.A. J. Phys. Chem. B 109, 2469 (2005).
[54] Allen, M.P.; Tildesley, D.J. Molecular Simulation of Liquids; Clarendon: Oxford, 1987.
[55] Hijón, C.; Español, P.; Vanden-Eijnden, E.; Delgado-Buscalioni, R. Faraday Discuss., 144, 301 (2010).
[56] Harmandaris, V.A. et al. Macromolecules 40, 7026 (2007).
[57] Harmandaris, V.A. and Kremer, K., Macromolecules 42, 4858 (2009).
[58] Harmandaris, V.A. and Kremer, K., Soft Matter 5, 3920 (2009).
[59] Likos, C.N. Phys. Rep. 348, 267 (2001).
[60] Gottwald, D.; Kahl, G.; Likos, C.N. J. Chem. Phys. 122, 204503 (2005).
[61] Bolhuis, P.G.; Chandler, D.; Dellago, D.; Geissler, P.G. Annu. Rev. Phys. Chem. 53, 291 (2002).
[62] Götze, W. in Liquids, Freezing and Glass Tranistion, ed. by J.-P. Hansen, D. Levesque, and J. Zinn-Justin (North-Holland, Amsterdam, 1991).