Soft matter is characterized by subtle forces and weak interactions, such as hydrogen bonds, van der Waals forces and π-π interactions that give rise to a complex hierarchy of organization. Soft matter organization under hard confinement (SOFT-CONFINE) can be fundamentally different from that obtained in thin films or in the bulk.1,2 Nanoporous hard templates provide a two-dimensionally confined space in which self-organization processes such as crystallization, protein secondary structure formation, mesophase formation and phase separation can be altered. A particular advantage of hard templates is that they provide a large parameter space (pore diameter, curvature, nature of pore walls) that can induce or manipulate self-assembly. These templates are particularly suitable for the rational generation of mesoscopic fine structures in the form of nanofibers because equilibrium and non-equilibrium states as well as a range of unprecedented confinement-induced morphologies with new and exciting properties can be realized. Understanding the self-assembly, thermodynamics and dynamics of soft materials under confinement will allow for their rational design as functional devices with tunable mechanical strength, processability, electronic and optical properties.
A principal focus of the proposed work is to find the basic underlying principles that give rise to directed self-assembly and controlled phase state in a range of soft materials under confinement. This ambitious but realistic approach requires a methodology that includes the synthesis of the hard templates, subsequent infiltration, surface functionalization and surface characterization as well as structural, thermodynamic and dynamical characterization in a number of soft materials with different type of interactions. These encompasscrystallizable polymers, amphiphilic molecules, liquid crystals and biopolymers with important potential applications (Scheme I). In addition, it requires the implementation of different but complementary techniques with high spatial and temporal resolution, operating over broad space and time scales. Earlier efforts employed track-etch polymer membranes and lead to inconclusive results due to lack of uniformity and stability.3 Herein we employ self-ordered nanoporous aluminum oxide (AAO) made by the electrochemical anodization of aluminum substrates as the inorganic model matrix that provides the required uniformity in diameter/length, thermal stability and resistance to organic solvents.4
In one area, crystallizable polymers are studied. Polymer crystallization under confinement can be substantially different from the bulk and this can have important technological applications for the design of polymeric nanofibers with tunable mechanical strength, processability and optical clarity. Important studies of polymer crystallization confined to droplets5,6 and within the spherical nanodomains of block copolymers7 emphasized the interplay between heterogeneous and homogeneous nucleation. We plan to extent these studies to polymer crystallization under hard confinement provided by model self-ordered AAO nanopores. Important open questions here are on the type of nucleation (homogeneous vs. heterogeneous), the size of critical nucleus, the crystal orientation and the possibility to control the overall crystallinity.8,9 Providing answers to these questions is of technological relevance for the understanding of nanocomposites containing semicrystalline polymers. The methodology includes a series of experiments (differential scanning calorimetry (DSC), X-ray scattering, polarizing optical microscopy (POM) and Infrared spectroscopy (FTIR)) aiming at the type of nucleation, critical nucleus size, crystal orientation, and the effect of dimensionality by employing a number of different semicrystalline polymers that include polyethylene (PE) and polyethylene oxide (PEO). The former (PE) will resolve an existing ambiguity in the literature8,9 whereas the latter (PEO) will facilitate the comparison with the crystallization within droplets.5,6
Confined self-assembly of amphiphilic molecules constitute a new route towards engineering novel nanostructures. Thus, in a second area, we expand the range of novel structures by employing amphiphilic molecules based on model block copolymers and semifluorinated alkanes. The former include the model poly(ethylene oxide-b-isoprene) (PEO-b-PI) diblock copolymer system with the very asymmetric phase diagram in the bulk.10 The combination of amorphous and crystallizable polymers in the same copolymer is expected to provide an unprecedented structural control over the possible nanostructured materials. It will further allow several studies of the effect of confinement/curvature/surfaces on (i) the stability of the bicontinuous phase, (ii) the possible suppression of the PEO crystallization below a certain pore diameter, (iii) the exact dependence of the domain- and interfacial- thicknesses on the effective pore diameter and (iv) the local (PEO, PI) and global (PI) chain dynamics under confinement. The second amphiphilic system is based on semifluorinated alkanes of the type: F(CF2)m(CH2)nH. Because of the strong amphiphilic character these molecular block copolymers have a rich phase diagram and exhibit solid-solid transitions below the melting temperature.11,12 However, the self-organization and dynamics of these model systems has not been explored under confinement.
In a third area, we propose to investigate confined liquid crystals. Understanding the physical properties of liquid crystals spatially confined to the nanometer scale is of fundamental and technological importance (liquid crystal display industry). From a fundamental side, surface anchoring of the orientation and wall-induced density modulations have length scales that compete with the length scales set by elasticity as well as by the bulk correlations.13,14 Hence, the equilibrium director field morphology, final optical and dielectric properties will depend on the elastic constants, surface coupling, size of the system and its density as well as the external field. Preliminary (unpublished) results from the current research team (RT) on the prototypical liquid crystal 5CB confined within self-ordered AAO revealed that all transition temperatures (nematic-to-isotropic, smectic A-to-nematic as well as the glass temperature) are affected (reduced) by confinement. Furthermore, the smectic phase is completely suppressed in AAO with pores having diameters below 35 nm, a finding that allows for estimating the critical nucleus size associated with the smectic A phase. Within this project, a number of different liquid crystal systems is investigated by DSC, X-rays, POM, FTIR and dielectric spectroscopy. First the effect of confinement is studied on the eutectic liquid crystal mixture E7 (a mixture of alkylcyanobiphenyls) in comparison to different alkylcyanobiphenyls including 5CB. Second, a number of different dipole-functionalized discotic liquid crystals (DLCs) based on hexa-peri-hexabenzocoronenes (HBCs) are investigated. Discotic liquid crystals of HBCs have recently been investigated with respect to the stability of phases15 and disk dynamics16 in view of the record high charge carrier mobility (molecular wires) for liquid crystals. It is well-documented that HBCs possess a range of length- and time-scales (i.e., they exhibit polymer-like behavior), however, it remains unknown how these scales are affected by the confinement.
Lastly, we propose to investigate the effect of confinement on the fundamental secondary motifs of polypeptides (α-helices and β-sheets, stabilized by intra- and inter-molecular hydrogen bonds, respectively).17 Inside AAO hard templates the intrinsic length scale of the peptide secondary structures compete with the extrinsic length scale of confinement.18 This may affect their type, coherence and dynamics with consequences on the performance of membrane configurations that are based on the functionality of biomolecules. Within this project we investigate the effect of confinement on the type and coherence of different peptide secondary structures. Initial experiments will focus on polypeptides bearing a single secondary structure (i.e., β-sheets of polyglycine (PGly), α-helices of poly(ε-carbobenzyloxy-L-lysine) (PZLL)) and proceed with polypeptides bearing both (polyalanine (Pala), as well as low molecular weight poly(γ-benzyl-L-glutamate (PBLG)). In a second step we investigate the effect of confinement on block copolypeptides based on PBLG-b-PGly19 and PBLG-b-Pala20 recently investigated in the bulk. This choice is based on the existence of multiple competing length scales: from the length scale of the peptide secondary structure, the length scale of nanophase separation between unlike blocks and the length scale set from the hard confinement that can influence the bulk phase state by producing unanticipated structures and phases.
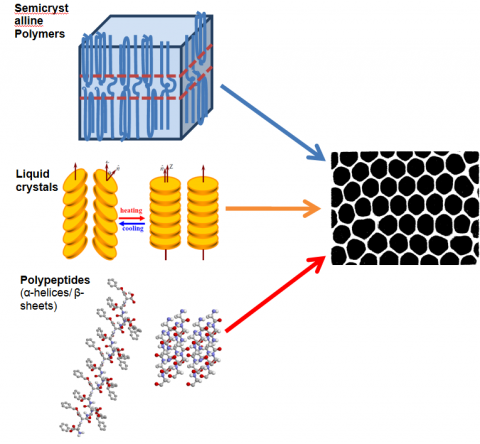
Scheme I. From semicrystalline polymers (top) to amphiphilic molecules, to liquid crystals (middle) and to polypeptides (bottom) confined within self-ordered nanoporous aluminum oxide (AAO). In all cases the investigation of the self-organization and dynamics under confinement, with important technological applications, is made for the first time to this extent.
The work is organized in work packages as follows:
WP1: Project coordination
WP2: Polymer crystallization under confinement
WP3: Confined self-organization of amphiphilic molecules
WP4: Nematic/smectic/discotic phases of liquid crystals under confinement and
WP5: Effect of confinement on the self-assembly and dynamics of biopolymers.
This interdisciplinary research -being at the forefront of the science of soft matter- will be carried out mainly at the University of Ioannina (UoI) by a group of researchers (2 Greek faculty members, 1 post-doc and 3 PhD students to be employed in the project). This core group is augmented by 3 scientists from abroad with expertise on the synthesis (M. Steinhart, University of Osnabrück; H. Duran, Max Planck Institute for Polymer Research, MPI-P) and surface characterization (H.-J. Butt (MPI-P)) of soft materials. The RT from the University of Ioannina (UoI) (G. Floudas (PI), P. Papadopoulos, 1 post-doc, 3PhD students), with expertise in molecular and macromolecular self-assembly and dynamics, undertake WP1-5. Within these WPs they investigate the self-assembly by thermodynamic (differential scanning calorimetry) and structural means (X-rays, polarizing optical microscopy, FTIR) as well as the dynamics (with dielectric spectroscopy) in crystallizable polymers, amphiphilic systems, liquid crystals and polypeptides under the hard confinement provided by nanoporous aluminum oxide templates. They investigate the effect of confinement/curvature on (i) the type of nucleation (homogeneous/heterogeneous) and overall crystallinity in crystallizable polymers, (ii) generating new nanostructures in amphiphilic molecules, (iii) the stability and coherence of smectic/nematic/discotic liquid crystalline phases and (iv) the self-organization and dynamics of polypeptides. Within these WP they collaborate closely with the UoO (M. Steinhart) where the electrochemical anodization of aluminum substrates as well as part of the self-organization under confinement and with the MPI-P (H. Duran, H.-J. Butt) where the infiltration, surface modification and surface characterization of soft materials.
The experimental results will have immediate academic and technological exploitation. From the pure academic side, research oncrystallizable polymers under confinement, will lead to the understanding of the role of confinement/surfaces on the type of nucleation and overall crystallinity. From a technological side, the research results and methodology could be implemented by Industry for the design of polymeric nanofibers with tunable mechanical strength, processability and optical clarity. In the amphiphilic systems, the possibility to engineer novel nanostructures could be exploited for the fabrication of inorganic nanostructured materials. In another area, understanding the physical properties of confined liquid crystals to the nanometer scale has fundamental interest for exploring the stability and persistence of smectic/nematic liquid crystal phases with technological relevance in the liquid crystal display industry and possibly in the design of DLCs with optimized charge carrier mobilities in field effect transistors (FTE) and in photovoltaic devices. Lastly, biopolymers under confinement can exhibit different secondary structures and dynamics with importance in the design of membranes based on the functionality of biopolymers located in nanopores.
The dissemination of results will be achieved with publications in highly ranked international journals, presentations in national and international conferences, the organization of an international conference at the UoI as well as with presentations of the pertinent findings to Industrial collaborators. Furthermore, the proposed research is important for the career development of 3 young researchers and 1 post-doc both in Academia and in Industry. For one, the integrated nature of the research (spanning synthesis, surface characterization, structure analysis and control, property measurement, all leading to the elucidation of governing structure-property relationships) invariably broadens the background of students and better enables them to set up a similarly integrated laboratory at the outset of their independent careers.
[1] Shin, K.; Xiang, H.; Moon, S.I.; Kim, T.; McCarthy, T.J.; Russell, T.P. Science 2004, 306, 76.
[2] Stainhart, M. Adv. Polym. Sci. 2008, 220, 123.
[3] Crawford, G.P.; Steele, L.M.; Ondriscrawford, R. et al. J. Chem. Phys. 1992, 96, 7788.
[4] Masuda, H.; Fukuda, K. Science 1995, 268, 1466.
[5] Massa, M.V.; Dalnoki-Veress, K. Phys. Rev. Lett. 2004, 92, 255509-1.
[6] Röttele, A.; Thurn-Albrecht, T.; Sommer, J.-U.; Reiter, G. Macromolecules 2003, 36, 1257-1260.
[7] Loo, Y.-L.; Register, R.A.; Ryan, A.J. Phys. Rev. Lett. 2000, 84, 4120-4123.
[8] Woo, E.; Huh, J.; Jeong, Y.G.; Shin, K. Phys. Rev. Lett. 2007, 98, 136103-1.
[9] Duran, H.; Stainhart, M.; H.-J. Butt, Floudas, G. Nano Lett. 2011, 11, 1671.
[10] Floudas, G.; Vazaiou, B.; Schipper, F.; Ulrich, R.; Wiesner, U. et al. Macromolecules 2001, 34, 2947.
[11] Russell, T.P.; Rabolt, J.F. Macromolecules 1986, 19, 1135.
[12] Nunez, E.; Clark, C.G.; Cheng, W.; Best, A. Floudas, G. et al. J. Phys. Chem. B 2008, 112, 6542.
[13] Wilms, D.; Winkler, A.; Virnau, P.; Binder, K. Phys. Rev. Lett. 2010, 105, 4.
[14] Schilling, T.; Frenkel, D., Phys. Rev. Lett. 2004, 92,8.
[15] Haase, N.; Grigoriadis, C.; Butt, H.-J.; Müllen, K.; Floudas, G. J. Phys. Chem. B 2011, 115, 5807.
[16] Elmahdy, M.M.; Floudas, G.; Mondeshki, M.; Spiess, H.W. et al. Phys. Rev. Lett. 2008, 100, 107801.
[17] Floudas, G.; Spiess, H.W. Macromol. Rapid. Commun. 2009, 30, 278.
[18] Duran, H.; Gitsas, A.; Floudas, G. et al. Macromolecules 2009, 42, 2881.
[19] Papadopoulos, P.; Floudas, G.; Schnell, I. et al. Biomacromolecules 2005, 6, 2352.
[20] Gitsas, A.; Floudas, G.; Mondeshki, M.; Spiess, H.W. Macromolecules 2008, 41, 8072.
Conclusions .
SOFT-CONFINE is positioned at the interface of well-established hitherto not cross-fertilizing fields with strong impact to nanocomposites, display industry, microelectronics and the biological world. It involves novel fabrication and characterization strategies that will enable us to achieve a fundamental understanding of the effect of confinement in controlling nucleation, crystal orientation and growth, the associated phase and phase transitions, and the dynamics of model soft materials that include crystallizable polymers, amphiphilic molecules, liquid crystals and biopolymers. This unified research approach will open up new routes for both scientific and technological research by tackling simultaneously structural, thermodynamic and dynamic issues over a broad spatio-temporal range. The research program will be implemented at the UoI by an interdisciplinary team headed by the PI, 1 staff scientist, 3 PhD students, 1 post-doc and 3 leading scientists from abroad with expertise in the fields of nanoporous materials and surface characterization. We envisage significantly contributing to the new emerging field of soft matter under hard confinement, where the many outstanding challenges are only now just being addressed.
References .
[1] Shin, K.; Xiang, H.; Moon, S.I.; Kim, T.; McCarthy, T.J.; Russell, T.P. Science 2004, 306, 76.
[2] Stainhart, M. Adv. Polym. Sci. 2008, 220, 123.
[3] Martin, C.R. Science 1994, 266, 1961.
[4] Cai, Z.; Martin, C. R. J. Am. Chem. Soc. 1989, 111, 4138.
[5] Liang, W.; C. R. Martin, C. R. J. Am. Chem. Soc. 1990, 112, 9666.
[6] Hou, S. F.; Wang, J. H.; Martin, C. R. Nano Lett. 2005, 5, 231.
[7] Lakshmi, B. B.; Martin, C. R. Nature 1997, 388, 758.
[8] Lee, S. B.; Mitchell, D. T.; Trofin, L. et al. Science 2002, 296, 2198.
[9] Dai, J. H.; Baker, G. L.; Bruening, M. L. Anal. Chem. 2006, 78, 135.
[10] Kohli, P.; Harrell, C. C.; Cao, Z. H.; Gasparac, R., Tan, W. H.; Martin, C. R. Science 2004, 305, 984.
[11] Yu, B.; Sun, P.; Chen, T.; Jin, Q.; Ding, D.; Li, B.; Shi, A.-C. Phys. Rev. Lett. 2006, 96, 138306.
[12] Yu, B.; Sun, P.; Chen, T.; Jin, Q.; Ding, D.; Li, B.; Shi, A.-C. J. Chem. Phys. 2007, 127, 114906.
[13] Crawford, G.P.; Steele, L.M.; Ondriscrawford, R. et al. J. Chem. Phys. 1992, 96, 7788.
[14] Masuda, H.; Fukuda, K. Science 1995, 268, 1466.
[15] Schmelzer, J.W.P.; Abyzov, A.S. J. Chem. Phys. 2011, 134, 054511-1.
[16] Wang, H.; Keum, J.K.; Heltner, A. et al. Science 2009, 323, 757.
[17] Ma, Y.; Hu, W.; Hobbs, J.; Reiter, G. Soft Matter 2008, 4, 540-543.
[18] Steinhart, M.; Göring, P.; Dernaika, H. et al. Phys. Rev. Lett. 2006, 97, 027801-1.
[19] Massa, M.V.; Dalnoki-Veress, K. Phys. Rev. Lett. 2004, 92, 255509-1.
[20] Röttele, A.; Thurn-Albrecht, T.; Sommer, J.-U.; Reiter, G. Macromolecules 2003, 36, 1257-1260.
[21] Loo, Y.-L.; Register, R.A.; Ryan, A.J. Phys. Rev. Lett. 2000, 84, 4120-4123.
[22] Woo, E.; Huh, J.; Jeong, Y.G.; Shin, K. Phys. Rev. Lett. 2007, 98, 136103-1.
[23] Duran, H.; Stainhart, M.; H.-J. Butt, Floudas, G. Nano Lett. 2011, 11, 1671.
[24] Jin, Y.; Rogunova, M.; Hiltner, A. et al. Polym. Sci., Polym. Phys. Ed. 2004, 42, 3380-3396.
[25] Block Copolymers, Hadjichristidis, N.; Pispas, S.; Floudas, G. J. Wiley and Sons Inc. 2002.
[26] Li, W.; Wickham, R.A. Macromolecules 2006, 39, 8492.
[27] Li, W.; Wickham, R.A.; Garbary, R.A. Macromolecules 2006, 39, 806.
[28] Erukhimovich, I.; Johner, A. EPL 2007, 79, 56004.
[29] Xiang, H.; Shin, K.; Kim, T.; Moon, S.I.; McCarthy, T.J.; Russell, T.P. Macromolecules 2004, 37, 5660.
[30] Xiang, H.; Shin, K.; Kim, T.; Moon, S.I. et al. J. Polym. Sci., Polym. Phys. 2005, 43, 3377.
[31] Sun, Y.M.; Steinhart, M.; Zschech, D. et al. Macromol. Rapid. Commun. 2005, 26, 369.
[32] Floudas, G.; Vazaiou, B.; Schipper, F.; Ulrich, R.; Wiesner, U. et al. Macromolecules 2001, 34, 2947.
[33] Russell, T.P.; Rabolt, J.F. Macromolecules 1986, 19, 1135.
[34] Nunez, E.; Clark, C.G.; Cheng, W.; Best, A. Floudas, G. et al. J. Phys. Chem. B 2008, 112, 6542.
[35] Wilms, D.; Winkler, A.; Virnau, P.; Binder, K. Phys. Rev. Lett. 2010, 105, 4.
[36] Schilling, T.; Frenkel, D., Phys. Rev. Lett. 2004, 92,8.
[37] Rozanski, S. A.; Stannarius, R.; Groothues, H.; Kremer, F. Liq. Cryst. 1996, 20, 59.
[38] Iannacchione, G. S.; Finotello, D., Phys. Rev. Lett. 1992, 69, 2094.
[39] Grigoriadis, C.; Duran, H.; Steinhart, M.; Butt, H.-J.; Floudas, G. in preparation.
[40] Haase, N.; Grigoriadis, C.; Butt, H.-J.; Müllen, K.; Floudas, G. J. Phys. Chem. B 2011, 115, 5807.
[41] Floudas, G.; Spiess, H.W. Macromol. Rapid. Commun. 2009, 30, 278.
[42] Duran, H.; Gitsas, A.; Floudas, G. et al. Macromolecules 2009, 42, 2881.
[43] Papadopoulos, P.; Floudas, G.; Schnell, I. et al. Biomacromolecules 2005, 6, 2352.
[44] Gitsas, A.; Floudas, G.; Mondeshki, M.; Spiess, H.W. Macromolecules 2008, 41, 8072.